Mass Formula Chemistry
The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180156 gmol. Expressed as a formula the mass fraction is.

Molar Mass Definition Formula In 2022 Molar Mass Chemistry Basics Relative Atomic Mass
Mass-Mole Calculations nmM Chemistry Tutorial.

. Write the equation at the beginning of every problem. Gypsum is a hydrate with two water molecules present for every formula. The formula mass and molecular mass of water H 2 O are one and the same while the formula and molecular mass of glucose are different from each other.
You must multiply by 100 at the end to express the value as a percentage. Group intranet Restricted permissions Barrow Group photos Restricted permissions Group calendar Restricted permissions Calculators. Percentage of mass solutes mass mass of solution x 100.
In chemistry the mass fraction of a substance within a mixture is the ratio alternatively denoted of the mass of that substance to the total mass of the mixture. Mass fraction can also be expressed with a denominator of 100 as. Because the individual masses of the ingredients of a mixture sum to their mass fractions sum to unity.
It is 5844 g mol 1. The molar mass links the mass of a substance to its moles. In chemistry the mass concentration ρ i or γ i is defined as the mass of a constituent m i divided by the volume of the mixture V.
This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in Earths atmosphere and crust. In 1912 Max Planck published the first journal article to describe the discontinuous emission of radiation based on the discrete quanta of energy. The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the.
Thus the mass concentration of a component in a mixture can be called the density of a component in a mixture. The basic formula for mass percent of a compound is. Mass percent mass of chemicaltotal mass of compound x 100.
During this article well learn the mass percent formula with various solved numerical. Because relative atomic weight is a unitless value relative formula mass technically. The mass formula is given as Mass ρ v.
The relative atomic mass of an element symbol A r is the relative mass of its atoms compared to the. The mass and weight of an object are not the same. Let me make it more clear with an example of sodium chloride.
PERCENT WATER IN A HYDRATE. Learn how to calculate the formula mass of a compound by adding the mass values of its atoms with BBC Bitesize GCSE Chemistry. The atomic mass unit can be related with the other mass unit by using the conversion factor1u 166054 X 10-24 g.
Petroleum and energy links. Solve out any chemical reaction using these formulas only from ClearIITMedical. The unit of molar mass is kgmol.
The molar mass M is a physical property and it is defined as the mass of one mole of the chemical substance or it is a ratio of the mass of a chemical compound to its amount of chemical substance. Atomic Mass of Elements The atomic mass of a few substances is tabulated below. Relative formula mass Atoms have very little mass so their relative atomic masses are used.
Mass percent mass of chemicaltotal mass of compound x 100. Given the equivalence of mass and energy expressed by. Mass percent components mass total mass x 100 or.
The solution means the atomic mass of the specific element. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. Of zero-point energy was developed by Max Planck in Germany in 1911 as a corrective term added to a zero-grounded formula developed in his original quantum theory in 1900.
The molar mass of sodium chloride is known. This explains the usage of ρ the lower case Greek. Hydrates are compounds that incorporate water molecules into their fundamental solid structure.
A related term you should know is relative formula mass relative formula weight. Get free list of Chemistry formulas online at ClearIITMedical. 1 mole of a pure substance has a mass equal to its molecular mass 1 expressed in grams.
174 convert the given mass of a substance to the amount of the substance in moles and vice versa by using the relative atomic or formula mass. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the. For a pure chemical the mass concentration equals its density mass divided by volume.
This is known as the molar mass M and has the units g mol-1 gmol grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. In a hydrate which usually has a specific crystalline form a defined number of water molecules are associated with each formula unit of the primary material.
If we have to measure one mole of sodium.
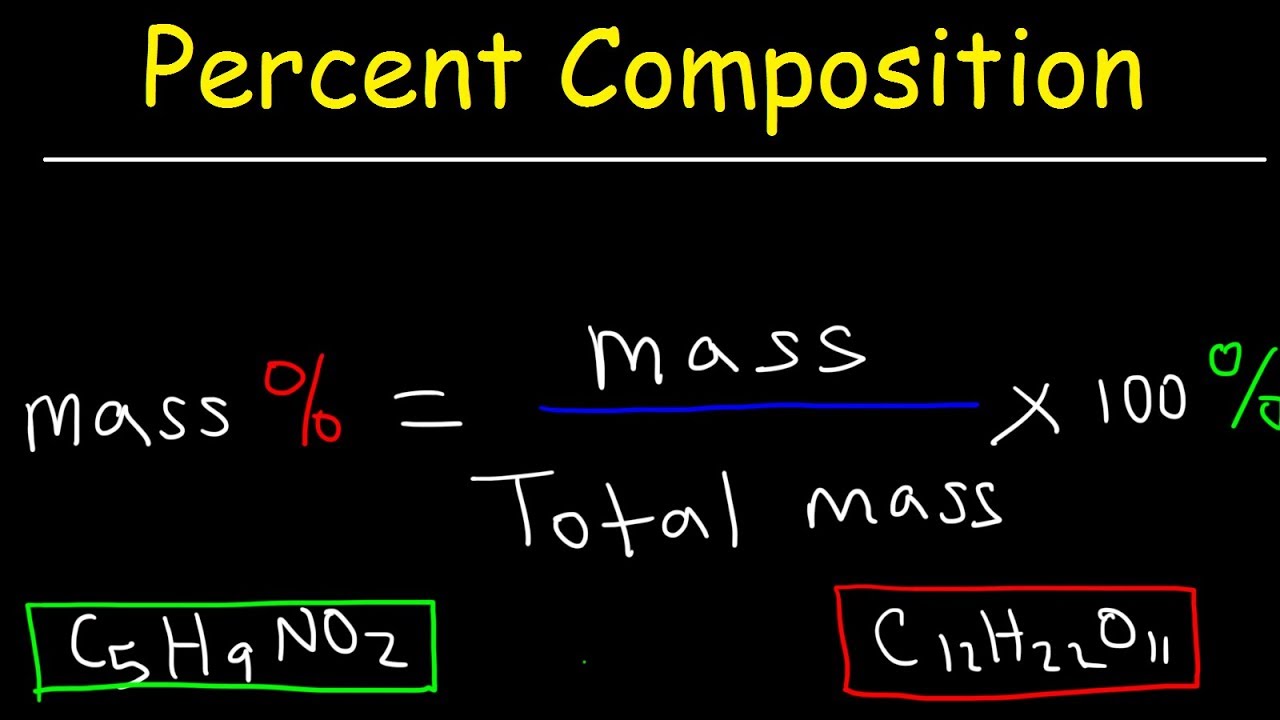
Chemistry Lesson Percent Composition Youtube Chemistry Lessons Teaching Chemistry Ap Chemistry

Difference Between Formula Mass And Molecular Mass Comparison Summary Teaching Chemistry Chemistry Lessons Chemistry Education

Grams To Moles Calculator Grams To Moles Molar Mass Math Methods

Formula Triangle Molarity Teaching Chemistry Chemistry Lessons Chemistry Classroom
No comments for "Mass Formula Chemistry"
Post a Comment